How are intracellular signals spatially controlled in neurons?
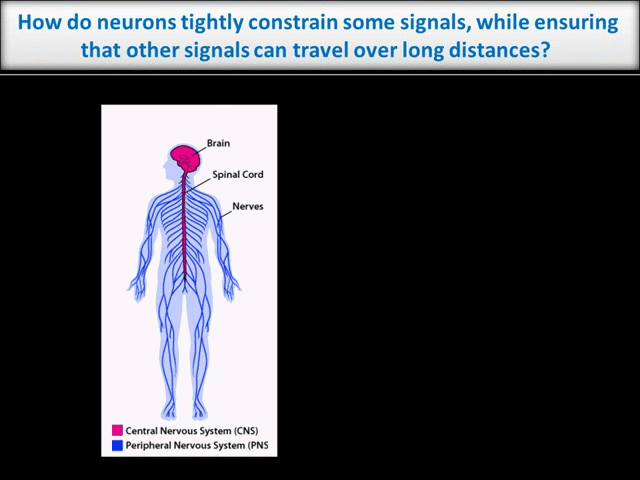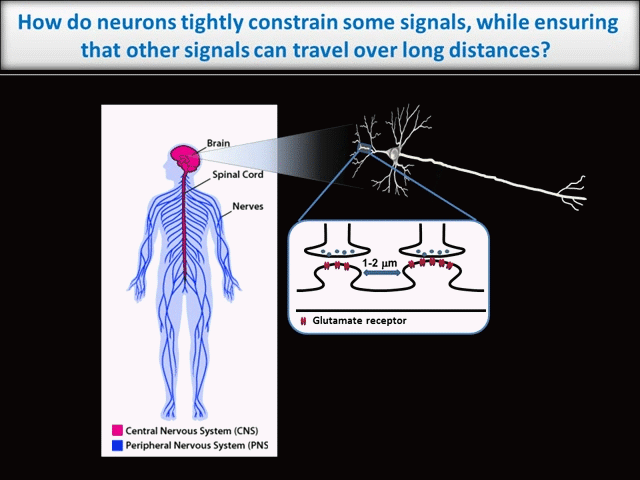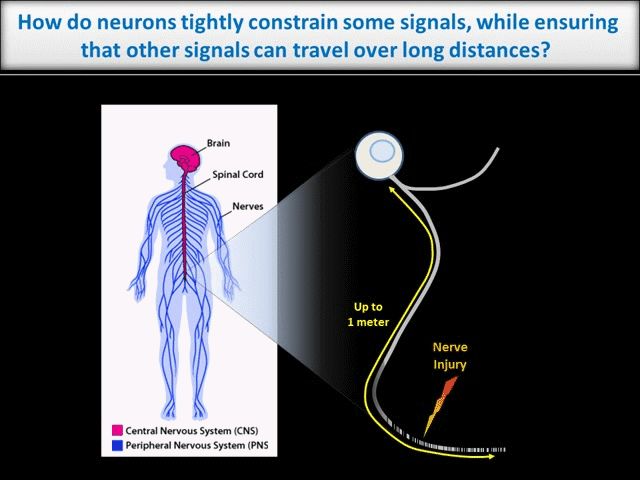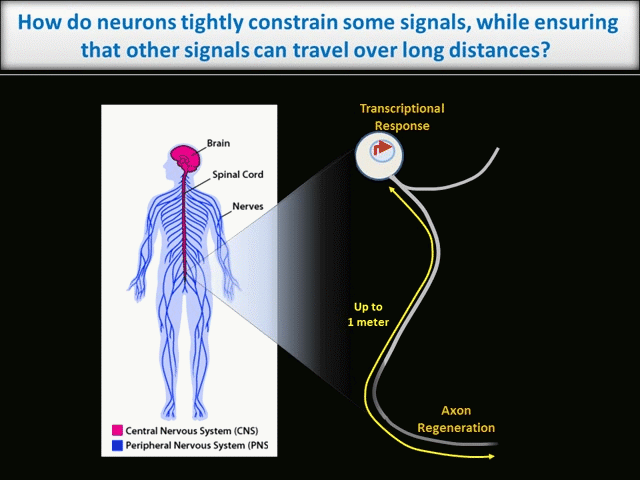
Every moment, our nervous system must sense, integrate and process vast amounts of information from the environment and then ensure that we act on that information appropriately. It is thus unsurprising that neurons, which must coordinate this huge array of inputs and outputs, are the most morphologically complex cells in the body. However, this morphological complexity presents a great challenge for the control of neuronal intracellular signals. For example, higher brain functions like learning and memory require precise regulation of individual synaptic connections between neurons in the Central Nervous System (CNS). These synapses may be separated from one another by only microns. In contrast, during development of the peripheral nervous system (PNS), and when adult PNS neurons respond to nerve injury, signals must be transferred over the vast distances that peripheral axons extend (up to 1 meter in humans – see schematic).
In the Thomas lab, much of our research focuses on how neurons meet these challenges, in large part because this is an interesting and exciting biological question. Equally importantly, though, breakdowns in the efficacy and fidelity of neuronal signaling are linked to a host of neurodevelopmental, neurodegenerative, and neuropsychiatric conditions, so insights into how intracellular signaling is coordinated in neurons may reveal new targets for therapy to ameliorate a range of devastating neuropathologies.
Palmitoylation-dependent Control of Neuronal Signaling
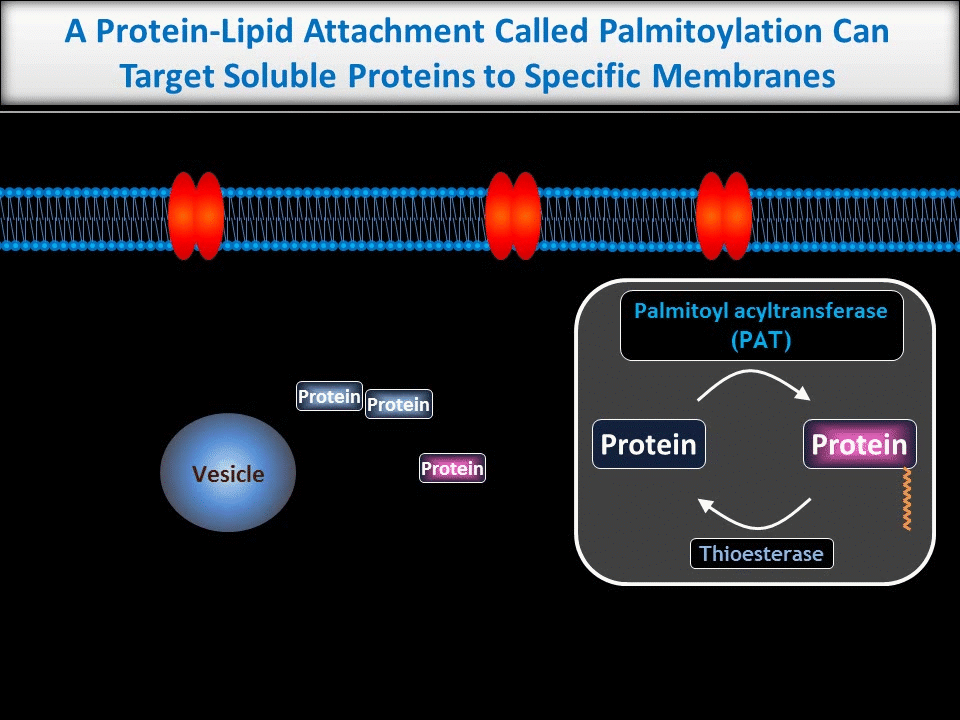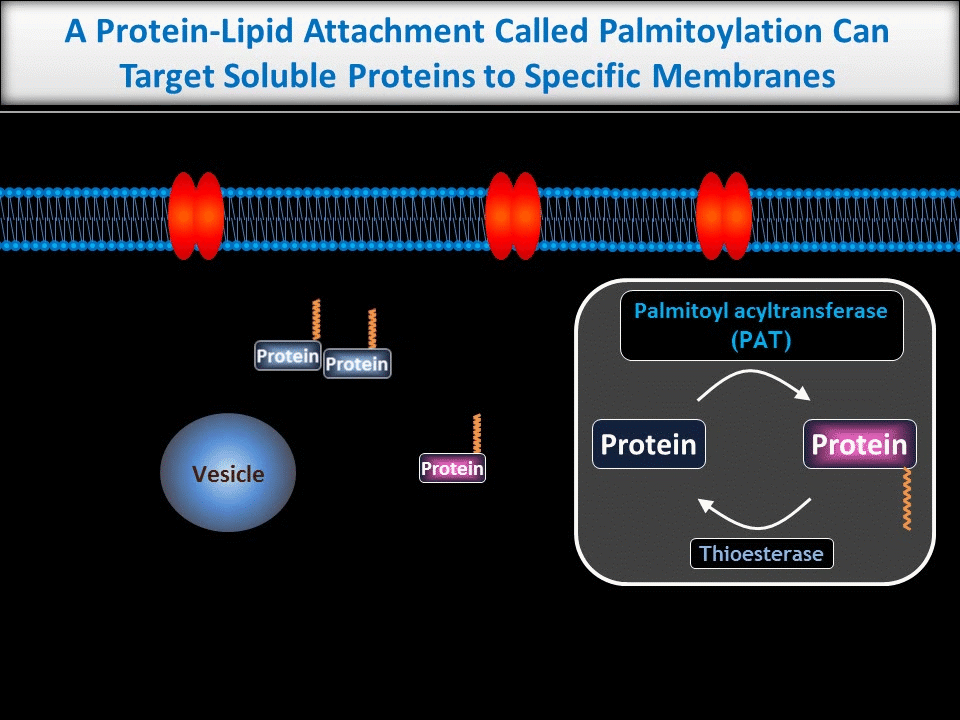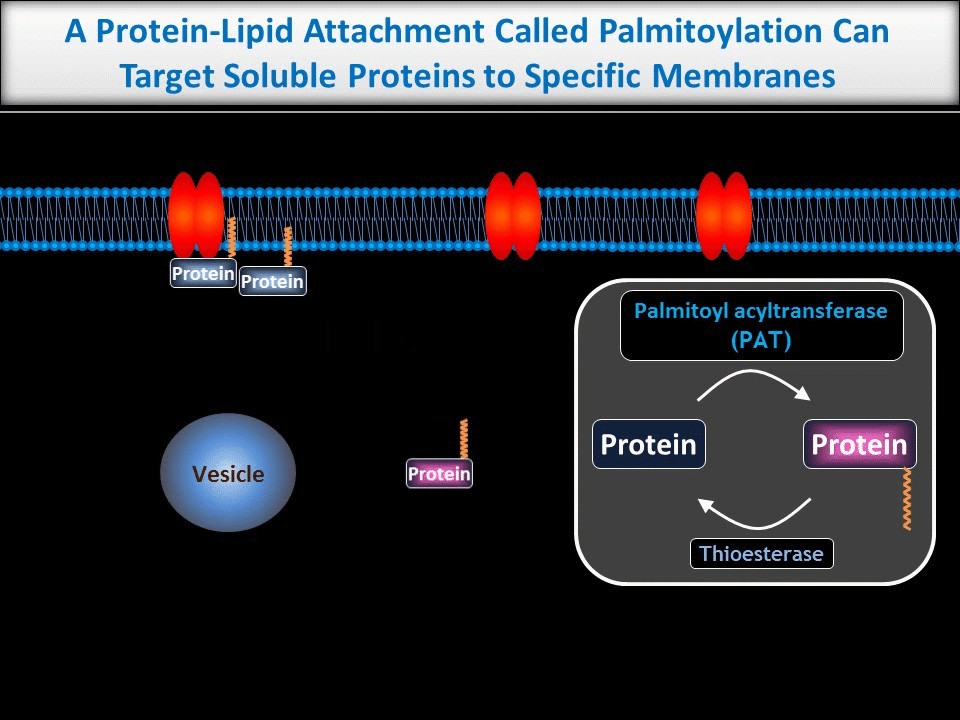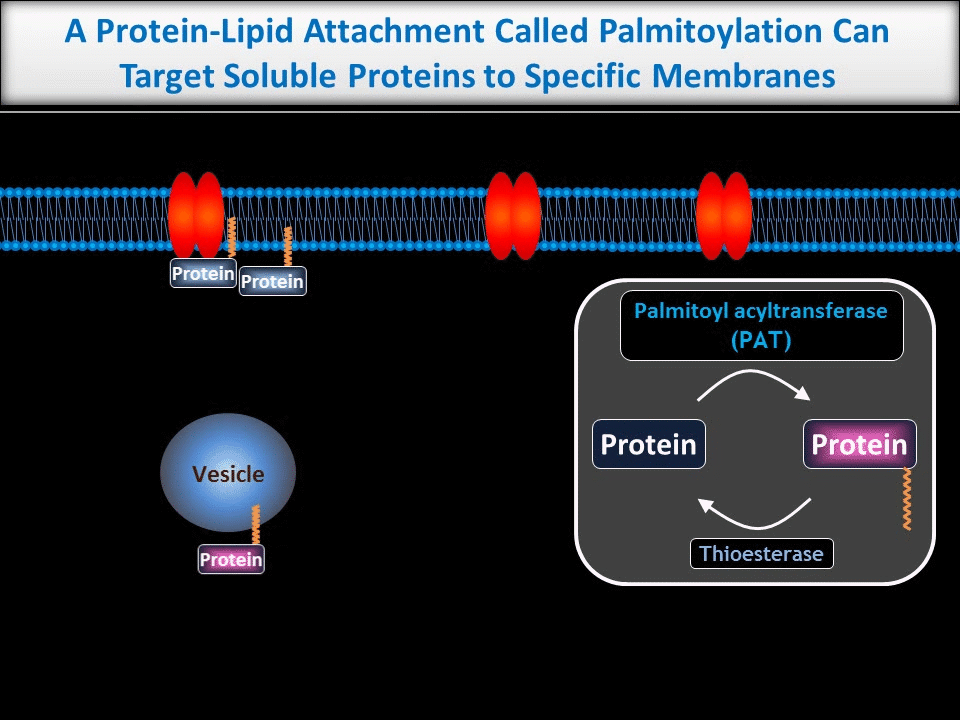
We are particularly interested in neuronal roles for palmitoylation, a protein-lipid modification that can target proteins to precise subcellular locations. We and others previously showed that palmitoylation is critical for appropriate targeting and trafficking of neurotransmitter receptors and several of their associated non-enzymatic ‘scaffold’ proteins. More recently, though, we have begun to reveal new roles for palmitoylation in the direct regulation of neuronal signaling enzymes.
Palmitoylation-dependent Regulation of Dendritic Spines
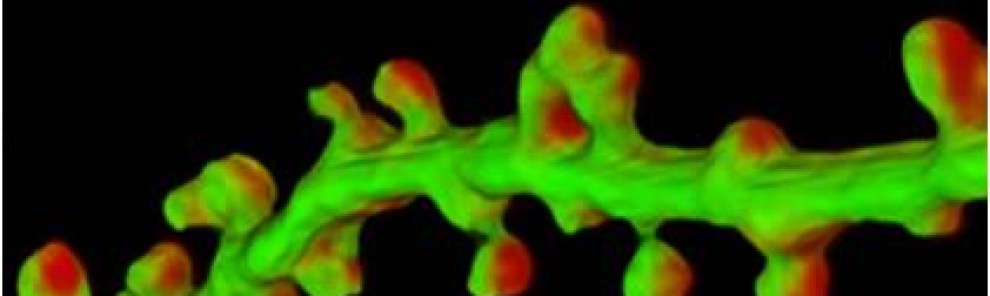
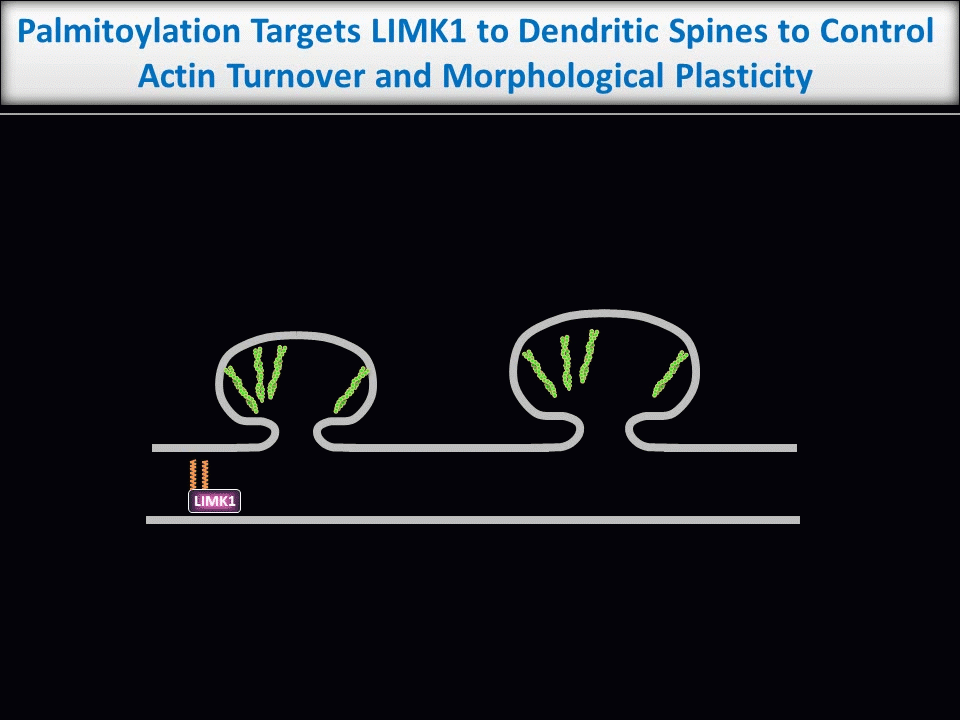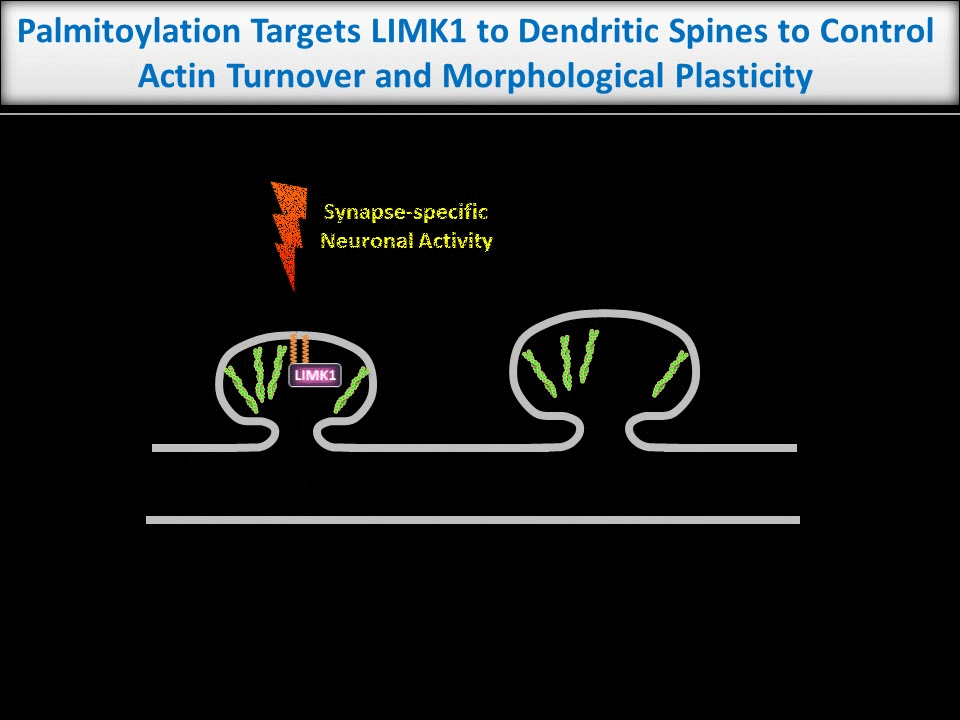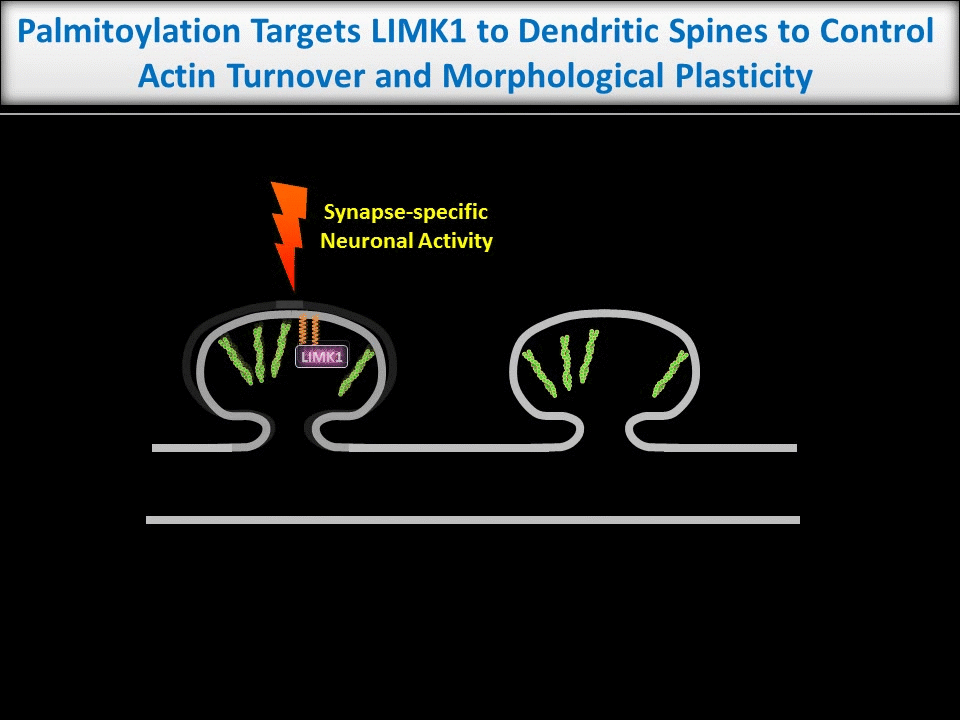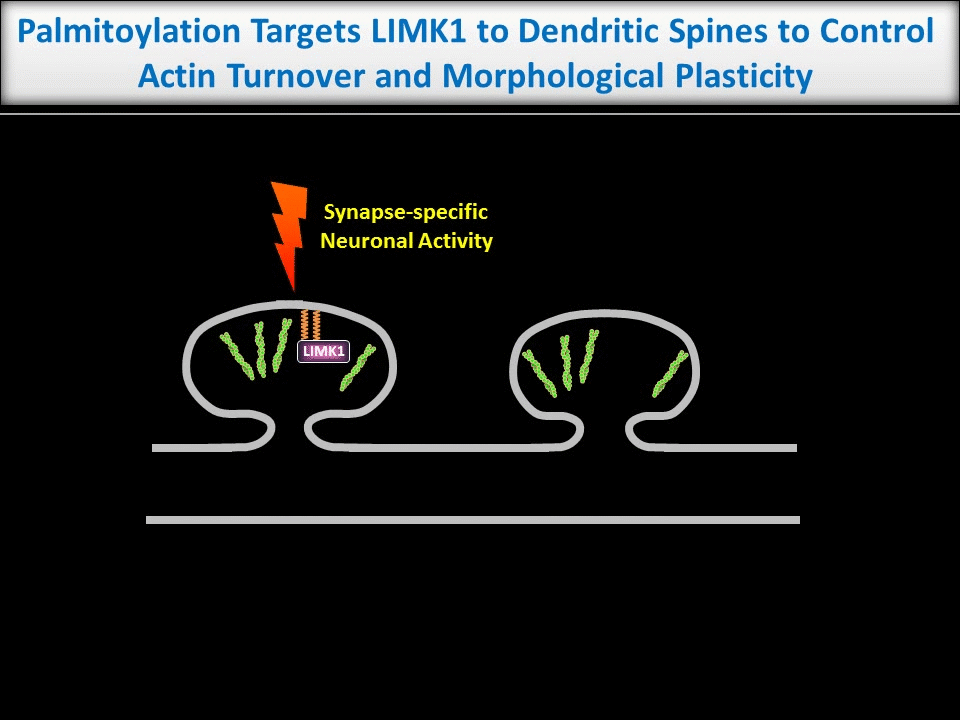
Dendritic spines are tiny structures on neuronal dendrites that are the site of most excitatory synapses. Changes in the size and shape of individual spines are closely associated with Long-term potentiation (LTP), a cellular correlate of learning and memory. Conversely, abnormal spine morphology and/or density are hallmarks of Intellectual Disability and other cognitive impairments, including Autism-Spectrum Disorders and schizophrenia. These findings suggest that precise regulation of dendritic spine morphology and number is critical for normal cognition. Spines are highly enriched in actin filaments, so changes in spine morphology likely require spatially precise actin regulation. We recently found that palmitoylation of the actin regulator LIM Kinase-1 (LIMK1) is critical for the control of actin polymerization in individual spines and, in collaboration with the lab of Dr Jean-Claude Beique, that palmitoyl-LIMK1 is required for the rapid changes in spine morphology that accompany LTP. These findings suggest that palmitoylation plays at least two key roles in spines, regulating the spine’s complement of neurotransmitter receptors and also its morphology.
New Roles for Palmitoylation in Axonal Retrograde Signaling
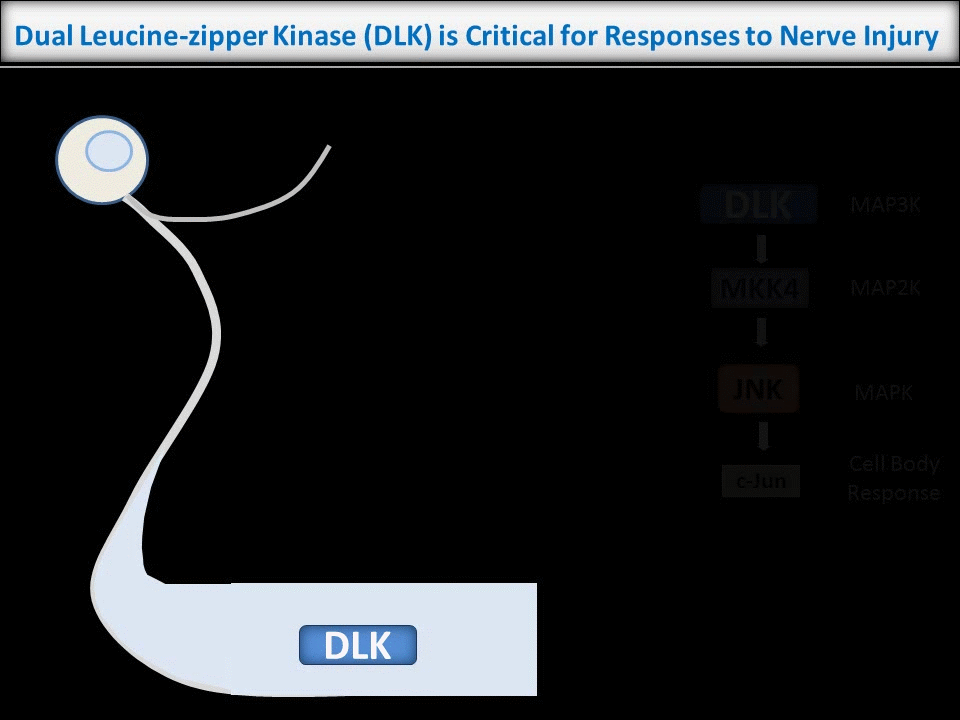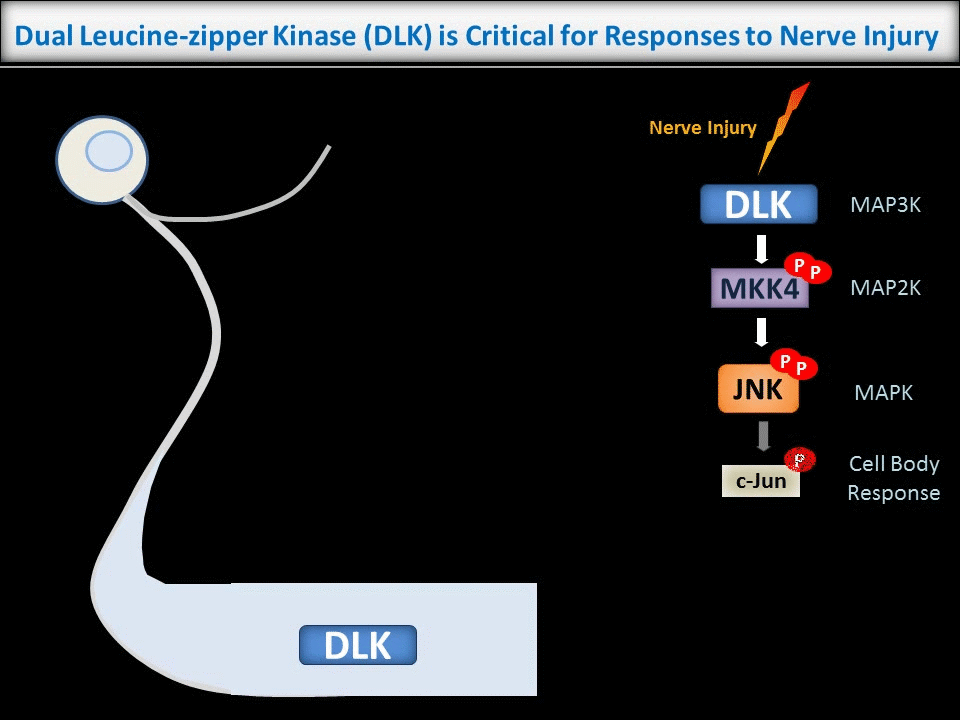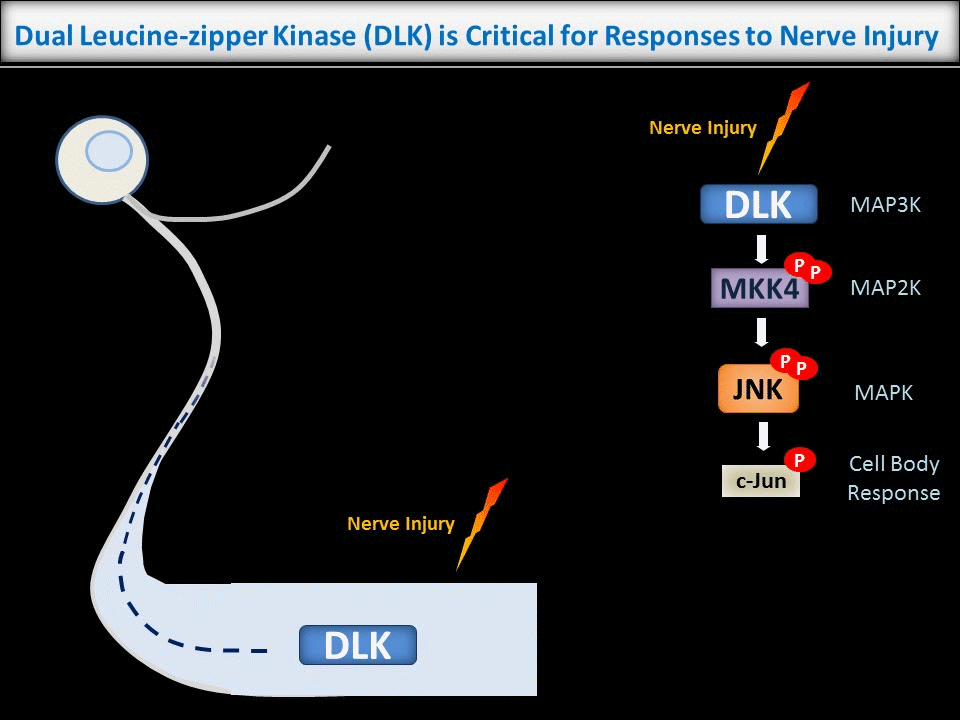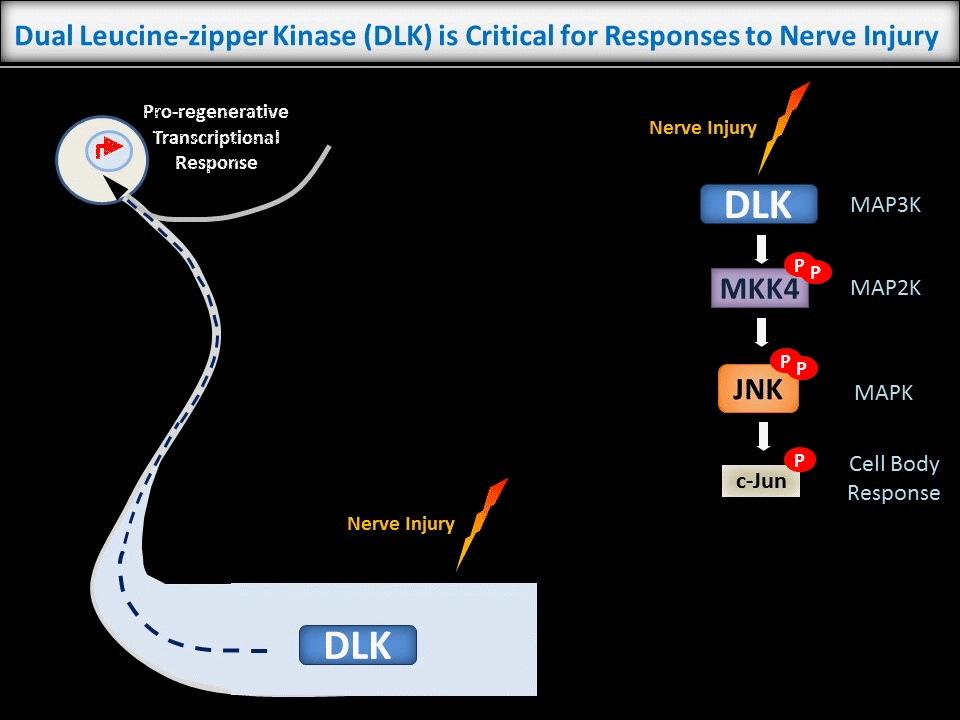
In contrast to the sub-micron regulation seen at CNS synapses, signals in PNS axons must often be conveyed over long distances. One key axonal signaling protein is Dual Leucine-zipper Kinase (DLK), which is critical to activate transcriptional responses to distal axonal injury. However, DLK is predicted to be a soluble, diffusible protein and it is thus unclear how DLK conveys long distance signals.
Interestingly, ongoing work in the lab suggests that palmitoylation acts in a previously unreported way, allowing DLK to hitchhike on axonal vesicles and thus convey these retrograde injury signals. To investigate this further, we are culturing neurons in microfluidic chambers, which allow physical segregation of cell bodies and distal axons.
More recently, we have expanded this work to address roles of palmitoylated proteins in in vivo models of neurodevelopmental and neurodegenerative disease. This work also takes advantage of the expertise of our close colleagues Drs. George Smith, Young-Jin Son and Shin Kang.